Institute of General and Experimental Biology, SB RAS, Ulan-Ude
Head of the Institute:
Vladimir M.Korsunov, Doctor of Biological Sciences
6 Sachyanova Str., Ulan-Ude, 670047, Russia
Tel.: (301 2) 331211 Fax: (301 2) 330134
email: bsc@buriatia.ru
Principal researchers:
B.B.Namsaraev, Ph.D., Head of the Laboratory of Microbiology
Gombo G.Gonchikov, Ph.D., Deputy Director
L.P.Kozireva, Ph.D.
S.M.Zakiyan, Ph.D., Deputy Director of the IC&G, SB RAS
T.I.Zemskaya, Ph.D., LIN SB RAS, sci. secretary
Project objectives
Establishment of the molecular (16S rRNA) philogeny of extremophils thermophilic, psychrophilic, halophilic, alkalophilic and haloalkalophilic bacteria, which were found in contrasting biotopes of the Baikal Region.
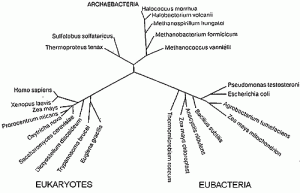
Over the past 20-30 years interest has grown for extremophilic Archaea and Eubacteria (Fig. 1), which is connected with ecologycal and biotechnologycal importance of these microorganisms.
Fig. 1. An unrooted phylogenetic tree, based on 16S rRNA sequences, illustrating the three primary lines of descent and some of their major subgroups (reproduced from WoÂse et al. (1990 )).
Background and significance of objectives
Authors of the project expect EEA to assist in the successful establishment of the phylogeny of Archaea Ë Eubacteria found in extremal biotopes (hot springs, mineral vents, salt and alkaline or soda lakes) based on sequence homology of 16S rRNA. To carrying out the work, highly skilled personnel of specialists will be trained.
During 1992-1999 investigations, the Baikal Region richness of extremal biotopes was determined. There are more than 200 hot springs with water temperature up to 70-79Ó— (for example in spring Baunt ñ up to 90Ó—), more than 100 soda-salt lakes with mineralisation up to 140 g/l and salt concentration 4%, and Õ from 8.5 to 11.4 (Table 1).
Table 1. Physico-chemical characteristic of extremal biotopes
|
Parameter |
Dimensions |
Baikal |
Hot springs |
Soda lake |
|
Temperature |
Ó— |
3,2-25 |
20ñ79 |
0-35 |
|
Salinity |
% |
0 |
0 |
0-4,0 |
|
Mineralisation |
g/l |
0,08-0,1 |
0,2-2,2 |
1-140 |
|
Acidity |
Õ |
6,8-8,2 |
6,8-10,2 |
8,5-11,4 |
The region feature is the location of extremal biotopes in immediate proximity and formation sharp change of phisico-chemical gradients. It is very interesting for isolation of new species of extremophils. Investigation of extremophils from contrasting biotopes was not conducted till now.
Investigation results found Archaea and Cyanobacteria in the composition of algae-bacterial mats formed in hot springs are present in Table 2.
Table 2. Extremophils in hot springs microbial mats (Western Zabaikalya)
|
Mats |
Tempera-ture, |
Õ |
— org., % |
Dominant |
|
Sulphuretum-1 |
30-71 |
8.7-9.3 |
3.1-16.6 |
Thiothrix, Thiobacillus |
|
Purple |
22-53 |
8.8-9.3 |
1.3-14.5 |
Thiocapsa roseopersicina, Chromatium venosum |
|
Green |
22-50 |
8.0-9.3 |
3.8-10.2 |
Chloroflexus auranticus |
|
Cyanobacte-rial |
20-54 |
8.9-9.8 |
13.8-19.8 |
Oscillatoria, Synechocystis Phormidium, Synechococcus |
|
Sulphuretum-2 |
20-36 |
7.8 |
4.8 |
Thioploca, Thiobacillus |
Table 3. Species bacterial diversity of lake Baikal
|
Species, (% of homology) |
1 |
2 |
3 |
Pseudomonas oleovorans |
w |
ph |
1 |
|
| Ps. Ambiqua |
w |
ph |
1 |
F. capsulatum |
w |
ph |
2 |
|
| Ps. Beijerinckii |
w |
ph |
1 |
F. aqualitica |
w |
ph |
2 |
|
| Ps. Aureofaciens |
w |
ph |
1,3 |
F. flavescens |
w |
ph |
4 |
|
| Ps. Synxaantha |
w |
ph |
1 |
Caulobacter crescentus (99%) |
w |
ph, Ï |
1,8 |
|
| Ps. Cohaerens |
w |
ph |
1 |
C. subvibroides (99%) |
w |
ph, Ï |
1,8 |
|
| Ps. Perolens |
w |
ph |
1 |
C. fusiformis |
w |
ph |
1 |
|
| Ps. Pseudomallei |
w |
ph |
1 |
C. bacteroides |
w |
ph |
1 |
|
| Ps. Eisenbergii |
w |
ph |
1 |
C. henricii |
w |
ph |
1 |
|
| Ps. Xanthe |
w |
ph |
1 |
C. vibroides |
w |
ph |
1 |
|
| Ps. Dysoxyli |
w |
ph |
1 |
C. leidyi |
w |
ph |
1 |
|
| Ps. Fragi |
w |
ph |
1 |
C. vibroides limonus |
w |
ph |
1 |
|
| Ps. Taetrolens |
w |
ph |
1 |
C. species |
w |
ph |
10 |
|
| Ps. Segnis |
w |
ph |
1 |
Brevibacterium eruthrogenes |
w |
ph |
1 |
|
| Ps. Incognita |
w |
ph |
1 |
B. fulvum |
w |
ph |
1 |
|
| Ps. Schuilkilliensis |
w |
ph |
4 |
B. quale |
w |
ph |
1 |
|
| Ps. Vesicatoria |
w |
ph |
4 |
B. lequmenticola |
w |
ph |
1 |
|
| Ps. Mendocina (96%) |
w |
à |
8 |
Micrococcus sp. |
w |
ph |
1 |
|
| Ps. Caryophillii (94%) |
w |
à |
8 |
M. luteus |
w |
ph |
1, 2,3 |
|
| Ps. Pickettii (100%) |
w |
à |
8 |
M. caseolyticus |
w |
ph |
1 |
|
| Ps. Testosteroni (100%) |
w |
à |
7 |
M. albscens |
w |
ph |
1 |
|
| Ps. Aeruginosa |
Ï |
ph, Ï |
2,6 |
M. flavescens |
w |
ph |
4 |
|
| Ps. Gracilus |
w |
ph |
4 |
M. sphaeroides |
w |
ph |
3,4 |
|
| Ps. Rubidinosa |
w |
ph |
4 |
M. varians |
w |
ph |
3 |
|
| Ps. Fluorescens |
w |
ph |
3,4 |
M. flavus |
w |
ph |
3 |
|
| Ps. Amlidua |
w |
ph |
3,4 |
M. coralloides |
w |
ph |
3 |
|
| Ps. Mycophaga |
w |
ph |
3,4 |
M. aquatilis |
w |
ph |
3 |
|
| Ps. Alcaligenes |
w |
ph |
3 |
Achromobacter≈urydice |
w |
ph |
1 |
|
| Ps. Desmolitic |
w |
ph |
3 |
A. parvulus |
w |
ph |
1 |
|
| Ps. Marginata |
w |
ph |
3 |
A. liguidum |
w |
ph |
4 |
|
| Ps. Liquefaciens |
w |
ph |
3 |
A. stoleniferum |
w |
ph |
4 |
|
| Ps. Lemoignei |
w |
ph |
3 |
A. album |
w |
ph |
4 |
|
| Ps. Faciens |
w |
ph |
3 |
A. lipolyticum |
w |
ph |
4 |
|
| Ps. Cepacia |
w |
ph |
2 |
A. desmolyticum |
w |
ph |
4 |
|
| Ps.stutzeri |
w |
ph |
2 |
A. inunctum |
w |
ph |
4 |
|
| Arthrobacter globiformis |
w |
ph |
1 |
Corynebacterium pseudodiphtheriticum |
w |
ph |
1 |
|
| A. simplex |
w |
ph |
1 |
C. hoaquii |
w |
ph |
1 |
|
| A. tumescens |
w |
ph |
1 |
C. michiganese |
w |
ph |
1 |
|
| A. oxydans |
w |
ph |
1 |
C. agropyri |
w |
ph |
1 |
|
| A. ureafaciens |
w |
ph |
1 |
Alcaligenes viscolactis |
w |
ph |
1 |
|
| Flavobacterium breve |
w |
ph |
1,3 |
A. marshalii |
w |
ph |
1 |
|
| F. solare |
w |
ph |
1 |
Acinetobacter sp. |
W |
ph |
1 |
|
| F. balustinum |
w |
ph |
1 |
A. calcoaceticus |
w |
ph |
3 |
|
| F. peregrinum |
w |
ph |
1 |
Sarcina flava |
w |
ph |
4 |
|
| F. suaveolens |
w |
ph |
1 |
C. flavescens |
w |
ph |
5 |
|
| F. tirrenicum |
w |
ph |
1 |
Myxococcus fulvus |
w |
ph |
5 |
|
| F. ferrugenium |
w |
ph, Ï |
1,3 8 |
M. virescens |
w |
ph |
5 |
|
| F. rigense |
w |
ph |
2 |
Sporocytofaga myxococcoides |
w |
ph |
5 |
|
| S. ventriculi |
w |
ph |
3 |
Cellfalcicula mucosa |
w |
ph |
5 |
|
| S.citrea |
w |
ph |
4 |
Promyxobacterium jonsonii |
w |
ph |
5 |
|
| Mycobacterium album |
w |
ph |
4 |
Methanosarcina barkeri |
s |
i |
9 |
|
| M. filiforme |
w |
ph |
4 |
M. mazei |
s |
i |
9 |
|
| Chromobacterium rubidium |
w |
ph |
4 |
Methanobacterium formicicum |
s |
i |
9 |
|
| C. lividum |
w |
ph |
2 |
M. thermoautotrophicum |
s |
i |
9 |
|
| Bacillus ruminatus |
w |
ph |
4 |
M. bryantii |
s |
i |
9 |
|
| B. amarificans |
w |
ph |
4 |
Methylosinus trichosporium |
s |
i |
10 |
|
| B. mycoides (99%) |
w |
ph, Ï |
4,7 |
Methylobacter bovis |
s |
i |
10 |
|
| B. mesentericus |
w |
ph |
4 |
M. capsulatus |
s |
i |
10 |
|
| B. macquariensis (87%) |
w |
Ï |
8 |
Methanomonas methanica |
s |
i |
10 |
|
| B. idosus |
‚ |
Ù |
4 |
Methylocystis echinoides |
s |
i |
10 |
|
| B.cereus |
w |
ph |
4 |
Desulfotomaculum guttoideum |
s |
i |
9 |
|
| B. larvae (87%) |
w |
ph |
4 |
Desulfovibrio desulfuricans |
s |
i |
9 |
|
| B. palustris |
w |
ph |
3 |
Clostridium sp. |
s |
mic |
9 |
|
| B. subtilis |
w |
ph |
2,3 |
Thioploca schmidlei |
Ï |
ph |
11 |
|
| B.pumilis |
w |
ph |
2,3 |
Xantomonas maltofilica |
Ï |
ph |
6 |
|
| B. circulans |
w |
ph |
2,3 |
Thiobacillus sp. |
Ï |
Ï |
6 |
|
| B. megaterium |
w |
ph |
2,3 |
Hyphomicrobium vulgare |
Ï |
Ï |
6 |
|
| B. salius |
w |
ph |
3 |
Lactobacillus lactis |
s |
ph |
2 |
|
| B. glutinosus |
w |
ph |
3 |
L. delbrueckii |
s |
ph |
2 |
|
| B. vitreus |
w |
ph |
3 |
Nitrosovibrio tenius (89%) |
w |
Ï |
8 |
|
| Paracoccus denitrificans |
w |
ph |
2 |
Sphaerotilus natans (96%) |
w |
Ï |
8 |
|
| Azotofix baicalensis |
s |
ph |
2 |
Citrobacter fruendii (98%) |
w |
Ï |
8 |
|
| Azotobacter chroococcum |
s |
ph |
2 |
Escherichia coli (100%) |
w |
Ï |
8 |
|
| Nocardia fastidiosa (88%) |
w |
Ï |
8 |
Myxococcus xantus (86%) |
w |
Ï |
8 |
|
| Flexibacter elegans (87%) |
w |
Ï |
8 |
Chondromyces crocatus (85%) |
w |
Ï |
8 |
|
| Sphingomonas yanoikuae (92%) |
w |
Ï |
8 |
Ehrlichia sennetsu (85%) |
w |
Ï |
8 |
|
| Rhodopseudomonas palustris (90%) |
w |
Ï |
8 |
Pirellula marina (86%) |
w |
Ï |
8 |
|
| Nitrosococcus mobilis (89%) |
w |
Ï |
8 |
Planctomyces maris (87%) |
w |
Ï |
8 |
|
| Cellvibrio ochraceus |
w |
ph |
5 |
Designations: 1 ñ source of isolation (w-water, s-sediments, Ï-mats); 2 ñ method of identification (ph ñ physiologo-biochemical, m ñ molecular-genetical, i ñ immuno-fluorescent, mic ñ microscopy); 3 ñ references: 1 ñ Lapteva, 1990; 2 ñ Verhozina, 1985; 3 ñ Parfenova, Illyaletdinov, 1985; 4 ñ Maksimova, Maksimov, 1989; 5 ñ Maksimova, Sergeeva, Maksimov, 1991; 6 ñ- Manakova et al., 1995; 7 ñ Belikov et al., 1996; 8 ñ Belkova et al., 1996; 9 ñ Namsaraev et al., 1995; 10 ñ authors data; 11 ñ Namsaraev, 1994).
Data about bacteria having the greatest phylogenetic likeness with 16S rRNA sequences present in Table 3.
The main objectives are:
- Comlectation the laboratory collection isolated and type species of Archaea and extremophilic Eubacteria (Halobacteriaceae, Methanosarcinaceae, Ectothiorhodospiriaceae, Bacillus (alk), Sulfolobus, Thermotoga, Methanococcus, Cyanobacteria, Rhodospirillum).
- Acquisition the laboratory equipment, instruments, materials (DNA-probes, kits, equipment for PCR, sequences rRNA etc.).
- Training of personal with EEA support.
- Collaboration with SB RAS Institutions and European scientists who work on these problems.
Expected results
It is expected to find typical for contrasting biotopes new phylogenetic subgroups of Archaea and extremophilic Eubacteria that are of ecological and biotechnologycal interest. Results obtained will facilitate collecting the extremophilic database; close scientific connections with home and foreign scientists and specialists in the field of biodiversity conservation and development of biotechnology.
List of publications of participants related to the project
-
Namsaraev B, Tarnueva E. Biodiversity of microorganisms and ecological processes in the hydroterms of Baikal Region // Abstr. Internat. Sympos. On Transect Studies on Global Change and Biodiversity. Beijng, China 6-8 May, 1996. ñ P. 100-101.
-
Belikov S.I., Grachev M.A., Zemskaya T.I. et al. Determination of taxonomic position of bacteria from Lake Baikal by sequencing 16S rRNA fragments // Microbiology. ñ 1996. ñ Vol. 65, π 6. ñ P. 855-864.
-
Namsaraev B.B., Kozireva L.P., Gorlenko V.M. et al. Bacterial formation of methane in alkaline lakes of Zabaikalya // Microbiology. ñ 1999 (in print).
-
Kozireva L.P., Dagurova O.P., Zemskaya T.I. Species diversity of microorganisms of Lake Baikal // Biodiversity in Baikalian Siberia ñ Nauka. Publ. House, SB, 1999 (in print).