SAVINKOVA L.K., SOKOLENKO A.A., RAU V.A., KOBZEV V.F., PONOMARENKO M.P., PONOMARENKO J.V., KOLCHANOV N.A.
Institute of Cytology and Genetics, Siberian Branch of the Russian Academy of Sciences, 10 Lavrentiev Ave., Novosibirsk, 630090, Russia
Keywords: TATA-binding protein affinnity, complementary duplexes, predicting
The values of the yeast TATA-binding protein (TBP) affinity for complementary duplexes of synthetic oligoDNA with the length of 15 bp were measured experimentally and analyzed by the computer system ACTIVITY. These values were found to correlate with the values of context-dependent properties: minor grove width and content of TA dinucleotides. An empirical equation for predicting the value of TBP/DNA affinity was suggested basing on these two correlations. The values of TBP/DNA affinity predicted by the equation suggested were shown to correlate with the values of TBP/DNA affinity, TBP/DNA bending, lifetime, and transcription activity for five sets of experimental data obtained by other authors.
Introduction
Functional properties of TATA-binding protein (TBP) have been most actively studied in a context of RNA polymerase II, where TBP recognizes and binds the conservative promoter element known as TATA box (Bucher, 1990). Binding of other basal transcription factors and RNA polymerase II, leading to formation of an active preinitiation complex, depends on the affinity of TBP interaction with TATA box (Buratowski, 1989, 1994; Sawadogo, 1990; Littie, 1989). The stability of the formed TBP/TATA-box complexes also affects the promoter strength in vivo and in vitro (Wobbe, 1990). Certain promoters of RNA polymerase II and the majority of promoters of RNA polymerases I and III contain no sequences displaying any apparent similarity to TATA box, although TBP is necessary for transcription initiation from these promoters too. In case of TATA-less promoters, TBP functions in combination with different sets of polypeptides, which assemble to DNA without any evident sequence specificity (Wingender, 1993; Conaway, 1993; Smale, 1990; Pugh, 1990). In certain cases, it is likely that TBP does not contact with DNA on such promoters or contacts in a different way than on the TATA-containing promoters. Presumably, other proteins are stabilizing the interaction of TBP with noncanonical DNA sequences (Wingender, 1993; Buratowski, 1994). The way TBP interacts with the canonical sequence of TATA box is interesting from the structural standpoint (Klug, 1993; Kim Y., 1993; Kim J., 1993; Jou, 1996). Unlike the other basal transcription factors and activator proteins interacting with DNA in its major groove (Latchman, 1995), TBP interacts through penetration of its amino acid residues between the bases of DNA minor groove (Klug, 1993; Kim Y., 1993; Kim J., 1993; Jou, 1996). Equilibrium dissociation constants of the complexes of yeast TBP with TATA-binding fragments of promoters, which characterize the TBP affinity for DNA, fall to a concentration range of 2-4 nM (Buratowski, 1988; Hoppes, 1992; Imbalzano, 1994; Hahn, 1989). A-T and T-A substitutions change the dissociation constants of TBP/DNA complexes 10-100-fold (Buratowski, 1988; Starr, 1995). Electrophoretic analysis has demonstrated that the stability of the TBP complexes with TATA-containing promoter regions correlates with the value of the DNA bending in the site of their binding: the more is the DNA bending, the longer is the lifetime of such complexes and the higher is the initiated transcription level (Starr, 1995). On the whole, the mechanism of the sequence-specificity of TBP recognition of and binding to DNA remains vague, despite the great volume of data on TBP/TATA interactions.
Table. Experimental data on the TBP affinity for synthetic 15-bp dsDNA and ssDNA
|
No |
Synthetic oligoDNA |
TBP/DNA affinity values, -ln[Kd] |
||
|
Strand I |
Strand II |
dsDNA |
ssDNA |
|
|
1 |
CGCCCTATAAAACCC |
GGGTTTTATAGGGCG |
23.54 |
24.23 |
|
2 |
CGGCTTATATAAGCC |
GGCTTATATAAGCCG |
21.59 |
21.06 |
|
3 |
GCGCCCTATACTACC |
GGTAGTATAGGGCGC |
23.61 |
24.08 |
|
4 |
GCGCCCTGTACTACC |
GGTAGTACAGGGCGC |
21.34 |
21.50 |
|
5 |
GCGCCCTATGCTACC |
GGTAGCATAGGGCGC |
20.97 |
21.17 |
|
6 |
CGCCCAAACCCTATA |
TATAGGGTTTGGGCG |
20.39 |
20.72 |
|
7 |
TATAAAACCCAGCGG |
CCGCTGGGTTTTATA |
16.53 |
17.73 |
|
8 |
GGTGGCTCACGCCTG |
CAGGCGTGAGCCACC |
16.22 |
16.27 |
|
9 |
AGAGTTCAAGACCTG |
CAGGTCTTGAACTCT |
16.16 |
15.76 |
|
10 |
CCCCCCCCCCCCCCC |
GGGGGGGGGGGGGGG |
11.63 |
11.78 |
Materials and Methods
Isolation and characterization of TBP were described in (Sokolenko, 1996). Oligodeoxyribonucleotides were synthesized by H-phosphate technique (Kumarev, 1992; Kobzev, 1997). Introduction of 32– to the 5′ end was carried out as described in (Sokolenko, 1996). Duplexes were produced by annealing complementary oligonucleotides supplemented in equimolar concentrations as described in (Kumarev, 1992; Kobzev, 1997). Formation of TBP/TATA-containing oligonucleotide complexes was carried out in buffer containing 50 mM Tris-HCl (pH 7.9), 7 mM MnCl2, 10 mM ![]() -MKE##, 250 m g/ml albumin, and 10% glycerin. The complexes formed were separated from free 32–-oligonucleotides on Sephadex G-50 or by gel retardation technique. To study the DNA-binding properties of TBP, curves of the dependence of the number TBP/oligonucleotide complexes formed upon the oligonucleotide concentrations were built. Oligonucleotide concentrations were varied in the range of 20 pM to 10 nM. The experimental data obtained were processed using the computer system ACTIVITY (Ponomarenko, 1997a) as described in (Ponomarenko, 1997b).
-MKE##, 250 m g/ml albumin, and 10% glycerin. The complexes formed were separated from free 32–-oligonucleotides on Sephadex G-50 or by gel retardation technique. To study the DNA-binding properties of TBP, curves of the dependence of the number TBP/oligonucleotide complexes formed upon the oligonucleotide concentrations were built. Oligonucleotide concentrations were varied in the range of 20 pM to 10 nM. The experimental data obtained were processed using the computer system ACTIVITY (Ponomarenko, 1997a) as described in (Ponomarenko, 1997b).
Results and discussion
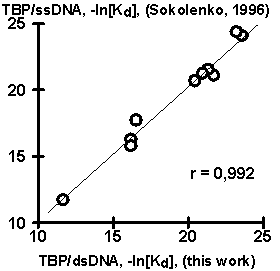
The experimental data on the values of the yeast TBP affinity for synthetic 15-bp dsDNA obtained as described above are listed in table. The rightmost column of the table includes also the corresponding values of the yeast TBP affinity for synthetic 15-bp ssDNA (strand I of the two complementary DNA strands studied herein), obtained earlier (Sokolenko, 1996). Comparison of the TBP affinities for ssDNA and dsDNA revealed unexpectedly their highly significant correlation (Fig. 1a). This is likely to indicate a certain similarity of the molecular mechanisms underlying TBP interaction with both single-stranded and double-stranded DNAs. However, additional experiments are required (and planned to be carried out later) to study this similarity in more detail.
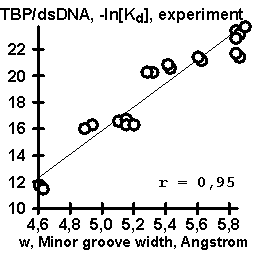
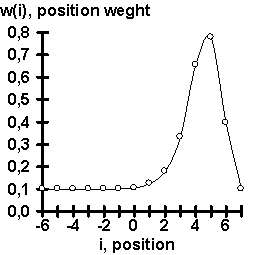
The experimental data on the TBP affinity for synthetic 15-bp dsDNA were also processed using the computer system ACTIVITY (Ponomarenko, 1997a, b). The results obtained are shown in Fig. 1b for the case of context-dependent conformational and physico-chemical properties; in Fig. 1c, for weighted concentrations of mono-. di, tri-, and tetranucleotides. In the first case (Fig. 1b), the linear correlation between the values of TBP/dsDNA affinity and the width of the DNA minor groove appeared to be most significant. This is in agreement with the X-ray structures of the TBP/dsDNA complexes (Kim Y, 1993; Kim J, 1993; Jou, 1996). In the second case (Fig. 1c), the most significant was the correlation between the TBP/dsDNA affinity and the content of the TA dinucleotides weighted using the weight function XTA,w, shown in Fig. 1d. Basing on these two correlations, we suggest an empirical equation (#) for predicting TBP/DNA affinity from the DNA sequence (Fig. 1e):
| TBP/DNA – Affinity = -35.13 + 10.21 x width – 0.72 x XTA,w | (#) |
In equation (#), the weight function is supplemented with a negative coefficient -0.72 despite the positive correlation of TBP/DNA affinity and the DNA content of TA dinucleotides (Fig. 1c). This is consistent with a commonly known but still unexplained fact that two symmetrical TBP domains bind to two different halves of TATA box: one domain, with TATA tetranucleotide; the other identical domain, with AAA tetranucleotide (Jou, 1996).
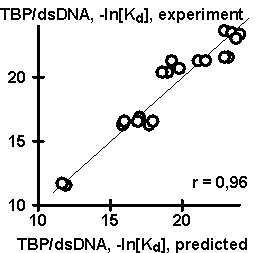
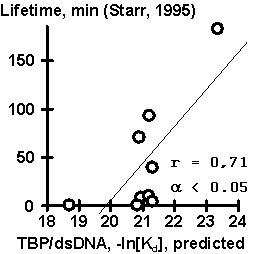
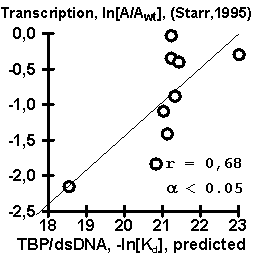
Equation (#) has been tested using independent experimental data, obtained earlier by other researchers. Results of the testing are shown in Fig. 2. The values of TBP/DNA affinity predicted using our equation appeared to correlate significantly with the data (a) of (Wiley, 1992), (b) on TBP/DNA bending, (c) on TBP/DNA lifetime, (d) on transcription activity obtained by (Starr, 1995), and (e) on transcription activity obtained by (Diagana, 1997). Thus, a simple equation (#) can be helpful in planning of a wide range of experiments
References
- Bucher P. (1990) – J. Mol. Biol., V. 212, P. 563-579
- Buratowski S., et al. (1988) – Nature, V. 334, P. 3742-3747
- Buratowski S., et al. (1989) – Cell., V. 56, P. 549-561
- Buratowski S. (1994) – Cell, V. 77, P. 1-3
- Conaway R., et al. (1993) – Annual Rev. Biochem., V. 62, P. 161-190
- Diagana et all (1997) – J. Mol. Biol., V. 265, P.480-493
- Hahn S., et al. (1989) – Proc. Nat. Acad. Sci. USA., V. 86, P. 5718-5722
- Hoppes B., et al. (1992) – J. Biol. Chem., V. 267, P. 11539-11547
- Imbalzano A., et al. (1994) – J. Biol. Chem., V. 269, P. 8280-8286
- Jou Z., et al. (1996) – J. Mol. Biol., V. 261, P. 239-254.
- Karas H., et al. (1996) – Comput. Appl. Biosci. V. 12, P. 441-446.
- Kim J., et al. (1993) – Nature., V. 365, P. 521-527
- Kim Y., et al. (1993) – Nature., V. 365, P. 512-520
- Klug A. (1993) – Nature, V. 365, P. 486-487
- Kobzev V.F., et al. (1997) Research & Applied Developments of SD RAS, N: IC&G SB RAS, P. 66-68.
- Kumarev V.P., at al. (1992) A method for the synthesis of oligo/poly/nucleotides and a device for its implementation, Author’s certificate of the USSR No. 1773916 of July 18,1992.
- Latchman D.S., (1995) Eukaryotic transcription factors. NY: Acad. Press. 387 P.
- Littie J.W. , et al. (1989) – Nature, V. 57, P. 103-113
- Ponomarenko M.P., et al. (1997a) – J. Comput. Biol., V. 4, P. 83-90.
- Ponomarenko M.P., et al. (1997b) – Mol
- Biol. (Msk), V. 31, P. 726-732.
- Pugh B.F., et al. (1990) – Cell. , V. 61, P. 1187-11-97
- Sawadogo M., et al. (1990) – Annual Rev. Biochem., V. 59, P. 711-754
- Smale S., et al. (1990) – Proc. Nat. Acad. Sci. USA., V. 87, P. 4509-4513
- Sokolenko A.A, et al. (1996) – Mol. Biol (Msk), V. 30, P. 279-285.
- Starr D., et al. (1995) – J. Mol. Biol., V. 236, P. 434-446
- Wiley (1992) – Proc. Nat. Acad. Sci. USA., V.89, P. 5814-5818
- Wingender E. (1993) Gene regulation in Eukaryotes. NY: VCH Weinheim. P. 75-84
- Wobbe C.R., et al. (1990) – Mol. Cell. Biol., V. 10, P. 3859-3867
| Figure 1. Results of the computer-assisted analysis of the experimental data (table) on the values of TBP affinity for complementary duplexes of synthetic 15-bp oligoDNA: (A) Highly significant correlation of the values of TBP affinity for ssDNA and dsDNA ; (B) Significant correlation of TBP affinity for ssDNA and a context-dependent property, widths of DNA minor groove (Karas, 1996); (C) Significant correlation of the TBP affinity for ssDNA and the content of dinucleotide TA weighted with the weight function (D) (for details, see Ponomarenko, 1997b); (E) Correlation of the experimentally measured TBP affinity for ssDNA and the corresponding affinity predicted using our empirical equation (#). |
| Figure 2. Results of the control testing of the empirical equation (#) using independent experimental data: (A) TBP/DNA affinity described in (Wiley, 1992); (B) TBP/DNA lifetime, (C) TBP/DNA bending, and (D) transcription activity described in (Starr, 1995); and (E) transcription activity described in (Diagana, 1997). |