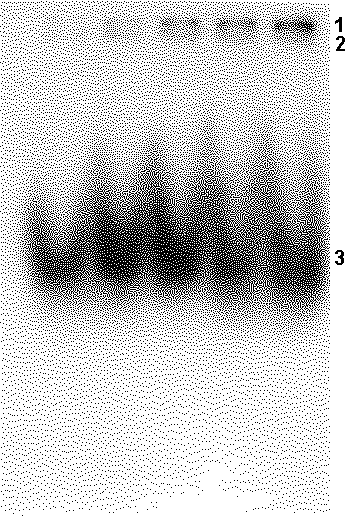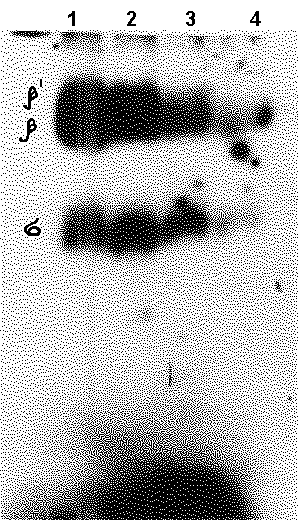SAVINKOVA L.K.+, SOKOLENKO A.A., SEDOVA V.M.1, RAU V.A., TULOKHONOV I.I, KOBZEV V.F., ARSHINOVA T.V.
Institute of Cytology and Genetics, Siberian Branch of the Russian Academy of Sciences, 10 Lavrentiev Ave., Novosibirsk, 630090, Russia;
1Institute of Cytology, Tihoretskogo 1, St. Petersburg, 194064, Russia;
+Corresponding author
Keywords: human, RNA polymerase II, holo-polymerase, core-polymerase, sigma subunit, TATA-binding protein, transcription, protein-protein interaction
Introduction
DNA-dependent RNA polymerases are complex proteins consisting of several polypeptides present in stoichiometric amounts. The catalytic function of the three different RNA polymerases is connected with a common structure consisting of two large and several smaller subunits. In order to work in a physiologically meaningful conditions, the enzymes need to possess the ability to participate in regulatory processes that determine where and when transcription is started and terminated. For this RNA polymerases interact not only with DNA and substrates but also with different transcription factors to modulate enzymic activity.
The progress has been achieved in a study of different steps in transcription to different subunits in E. coli RNA polymerase and fewer is known about the structure-functional relationships of the eukaryotic enzymes.
It is known, that E. coli RNA polymerase forms a relatively simple structure consisting of four core subunits – ![]() ‘,
‘, ![]() and 2
and 2![]() – and initiation factor s (conferring promoter specificity. It has been established in E.coli RNA polymerase that the assembly pathway of core polymerase is :
– and initiation factor s (conferring promoter specificity. It has been established in E.coli RNA polymerase that the assembly pathway of core polymerase is : ![]() –
– ![]() 2 –
2 – ![]() 2
2![]() -a 2
-a 2![]()
![]() ‘ (core enzyme, E) – E
‘ (core enzyme, E) – E![]() (holoenzyme) [1].
(holoenzyme) [1].
The assembly pathway of the eukaryotic homolog of E. coli RNA polymerase is not determined yet. During the last years attempts were made to study subunit structure of the eukaryotic enzymes and to compare it with the prokaryotic RNA polymerase structure.
As result, by sequencing the genes for the large subunits of yeast RNA polymerases II and III found regions of partial homology to the E. coli ![]() ‘-subunit [2]. Structural and evolutionary considerations suggest that some of five cysteine residues of the
‘-subunit [2]. Structural and evolutionary considerations suggest that some of five cysteine residues of the ![]() ‘-subunit are responsible for binding the zinc atom and they are conserved in nearly all prokaryotic RNA polymerases [3]. Treich et al. have been shown that the amino-terminal segments of the
‘-subunit are responsible for binding the zinc atom and they are conserved in nearly all prokaryotic RNA polymerases [3]. Treich et al. have been shown that the amino-terminal segments of the ![]() ‘ homologs of yeast RNA polymerase II and III also contain a cluster of cysteine residues, and these segments are capable of binding zinc too [4].
‘ homologs of yeast RNA polymerase II and III also contain a cluster of cysteine residues, and these segments are capable of binding zinc too [4].
The E.coli RNA polymerase ![]() subunit is known to contain the binding site for ribonucleotides and the catalytic site for phospfodiester bond formation [5].
subunit is known to contain the binding site for ribonucleotides and the catalytic site for phospfodiester bond formation [5].
A comparison of the nucleotide sequence of the gene coding for the large subunit of the Drosophila RNA polymerase II with E.coli ![]() -subunit showed a total of nine regions of homology [6].
-subunit showed a total of nine regions of homology [6].
It has been shown, the two large subunits of the yeast RNA polymerase II are homologous to the ![]() ‘- and
‘- and ![]() -subunits of the bacterial core enzyme [7,8]. So, the largest and the large eukaryotic RNA polymerase subunits are homologous to the
-subunits of the bacterial core enzyme [7,8]. So, the largest and the large eukaryotic RNA polymerase subunits are homologous to the ![]() ‘ and
‘ and ![]() subunits of E. coli RNA polymerase, respectively.
subunits of E. coli RNA polymerase, respectively.
![]() – and
– and ![]() ‘-subunits do not form stable complexes, but E. coli RNA polymerase a -subunit plays an essential roles in the core enzyme assembly and in transcription activation by class I factors [9].
‘-subunits do not form stable complexes, but E. coli RNA polymerase a -subunit plays an essential roles in the core enzyme assembly and in transcription activation by class I factors [9]. ![]() -related subunits in higher eukaryotes are also involved in the early step of enzyme assembly [10].
-related subunits in higher eukaryotes are also involved in the early step of enzyme assembly [10].
In E.coli RNA polymerase ![]() 70– subunit regulates the binding of RNA polymerase to promoters. Biochemical and genetics studies have established that dramatically decreases the affinity of the core enzyme for nonpromoter sequences [11] and increases the affinity of RNA polymerase for the promoter sequences. Like the E.coli core polymerase, mammalian RNA polymerase II found to bind stably and nonspecifically with free DNA [12, 13].
70– subunit regulates the binding of RNA polymerase to promoters. Biochemical and genetics studies have established that dramatically decreases the affinity of the core enzyme for nonpromoter sequences [11] and increases the affinity of RNA polymerase for the promoter sequences. Like the E.coli core polymerase, mammalian RNA polymerase II found to bind stably and nonspecifically with free DNA [12, 13].
As ![]() 70 subunit transforms the core polymerse into a sequence-specific DNA-binding protein by increasing its affinity for promoter sequences as transcription factor IIF (TF IIF or RAP 30/74) could be enhance the specificity with which RNA polymerase II recognizes and binds to thepreinitiation complex [14]. In this respect TF IIF functions as does the E.coli
70 subunit transforms the core polymerse into a sequence-specific DNA-binding protein by increasing its affinity for promoter sequences as transcription factor IIF (TF IIF or RAP 30/74) could be enhance the specificity with which RNA polymerase II recognizes and binds to thepreinitiation complex [14]. In this respect TF IIF functions as does the E.coli ![]() -subunit.
-subunit.
Sopta et al. [15] have shown that TFIIF possesses an RNA polymerase II-binding domain similar to the highly conserved RNA polymerase-binding domains present in region 2 of bacterial s factors. And they obtained evidence that TFIIF is capable of binding to both mammalian and E.coli RNA polymerases [16].
Thus, the results gather confirming a homology of structures and functions of the prokaryotic and eukaryotic transcription machines.
Results
On the basis of variety of evidence indicating the structural and functional conservatism of the common subunits for the eukaryotic RNA polymerase II and the bacterial RNA polymerase, we also investigated the possibility of human RNA polymerase II and E.coli holo-polymerase, core-polymerase and ![]() -subunit with TBP interaction.
-subunit with TBP interaction.
We showed the interaction of the human RNA polymerase II with TATA-binding protein (TBP). In those experiments, RNA polymerase II was preincubated with increasing concentrations of TBP, then added a fixed amount of the alkylating derivative of 32P-oligodeoxyribonucletide (oligoN) and incubation was continued. The resulting covalent complexses were assayed by electrophoresis in PAAG under denaturing conditions. As is seen from Fig. 1A and B, the alkylating 32P-oligoN form covalent complexes with the largest subunit of RNA polymerase II; the higher TBP concentrations, the lesser the number of such complexes.
Then we analysed a possibility of the interaction of TBP with E.coli ![]() -subunit, core polymerase and holo polymerase. For this purpose, we preincubated a constant amount of yeast TBP with core-polymerase (or -subunit) in increasing concentrations, and then added 32P-oligoN in fixed amount and continued incubation. We observed an increase in the number of the complexes of 32P-oligoN with core-polymerase (or s -subunit) – Fig.2, and constant number of the complexes of TBP with 32P-oligoN. This is shown by electrophoretic mobility-shift assay of 32P-oligoN binding by core-polymerase and TBP.
-subunit, core polymerase and holo polymerase. For this purpose, we preincubated a constant amount of yeast TBP with core-polymerase (or -subunit) in increasing concentrations, and then added 32P-oligoN in fixed amount and continued incubation. We observed an increase in the number of the complexes of 32P-oligoN with core-polymerase (or s -subunit) – Fig.2, and constant number of the complexes of TBP with 32P-oligoN. This is shown by electrophoretic mobility-shift assay of 32P-oligoN binding by core-polymerase and TBP.
So, TBP and core-polymerase (or TBP and s -subunit) do not interact each other.
Fig. 3 presents the results of ineraction E.coli holo-polymerase and TBP with alkylating derivative of the 32P-oligoN. As can be seen from the autoradiogram, the presence of TBP reduce the number of E.coli “RNA polymerase : 32P-oligoN” complexes proportionaly to its concentration. However. no band corresponding to a “TBP : 32P-oligoN” complex exposes, which supports the formation of a “TBP E.coli holo-polymerase” complex.
Conclusions
The data obtained on the interaction of human RNA polymerase II and E.coli holo-polymerase with TBP favor the hypothesis about functional homology between prokaryotic and eukaryotic RNA polymerases and that they may have had a common ancestor.
Analysis of the prokaryotic and eukaryotic transcription machinery, interspecific protein-protein and protein-nucleic acid interactions therefore is likely to shed new light on the molecular evolution of transcriptional apparatus in the cell of eukaryot. These investigations should provide understanding of the molecular mechanisms interaction between eukaryotic components of the transcription machinery.
We are very grateful Dr. H. Heumann and Dr. E. Zaychikov for gift of the recombinant core-polymerase and s -subunit.
This work is supported by grant of RFFR (97-04-49354).
References
- A. Ishihama, “Subunit assembly of E.coli RNA polymerase” Advan. Biophys. 14, 1 (1981)
- L.A. Allison, M. Moyle, M. Shales and C.J. Ingles, “Extensive homology among the largest subunits of eukaryotic and prokaryotic RNA polymerases” Cell 42, 599 (1985)
- K.J. Bergsland and R. Haselkorn, “Evolutionary relationships among eubacteria, cyanobacteria and chloroplasts: evidence from the rpo C1 gene of Anabaena sp. strain PCC 7120” J. Bacteriol. 173, 3446 (1991).
- I. Treich, M. Riva and A. Sentenac, “Zinc-binding subunits of yeast RNA polymerases” J. Biol. Chem. 266, 21971 (1991)
- S.A. Kumar, “The structure and mechanism of action of of bacterial DNA-dependent RNA polymerases” Progr. Biophys. Molec. Biol. 38, 165 (1981)
- D. Falkenburg, B. Dworniczak, D. Faust and E.K.F Bautz, “RNA polymerase II Drosophila relation of its 140 000 Mr subunit to the beta subunit E.coli RNA polymerase” J. Mol. Biol. 195, 929 (1987)
- D.W. Martindale, “A conjugation specific gene Tetrahymena encodes a protein homologous to yeast RNA polymerase subunits (RPB 3, RPC 40) and similar to a portion of the prokaryotic RNA polymerase subunit (rpo A)” Nucl. Acids. Res. 18, 2953 (1990)
- R.A. Young, “RNA polymerase II” Annu. Rev. Biochem. 60, 689 (1991)
- T. Negishi, N. Fujita and A. Ishihama, “Structural map of the alpha subunit of E.coli RNA polymerase: structural domains identified by proteolytic cleavage” J. Mol. Biol. 248, 723 (1995)
- Y. Yao, K. Yamamoto et al, “Mouse RNA polymerase I 16-kDa subunit able to associate with 40-kDa subunit is a homolog of yeast AC19 subunit of RNA polymerases I and III” J. Biochem. 271, 32881 (1996)
- D. Hinkle and M.J. Chamberline, “Studies of the binding of E.coli RNA polymerase to DNA. 1.The role of sigma subunit in site selection” J. Mol. Biol. 70, 157 (1972)
- Y. Li and R.D. Kornberg, “Interplay of positive and negative affectors in function of the C-terminal repeat domain of RNA polymerase II” Proc. Natl. Acad. Sci. USA 91, 2362 (1994)
- L.K. Savinkova, A.A. Sokolenko, V.V. Gorn et al, “Binding of the human RNA polymerase II with olideoxynucleotide identical regulatory elements eukaryotic promoter genes”Dokl. Acad. Nauk. SSSR 317, 1494 (1991)
- J.W. Conaway and R.C. Conaway, “An RNA polymerase II transcription factor shares functional properties with E.coli ” Science 248, 1550 (1990)
- M. Sopta, Z. Burton and J. Greenblatt, “Structure and associated DNA-helicase activity of a general transcription initiation factor that binds to RNA polymerase II” Nature 341, 410 (1989)
- S. McCracken and J. Greenblatt, “Related RNA polymerase-binding regions in human RAP 30/74 and E.coli ” Science 253, 900 (1991)
- A.A. Sokolenko, V.M. Sedova, I.I. Tulokhonov, L.K. Savinkova, “Interaction of the human RNA polymerase II with TATA-binding protein” Mol. Biol. (Rus.) 31, 963 (1997)